Research - Der Pharmacia Lettre ( 2021) Volume 13, Issue 9
The present study was to develop oral mucoadhesive drug delivery systems of metronidazole for an effective and safe treatment of Entamoeba histolytica. The microspheres were formulated by orifice-ionic gelation technique using chitosan, Sodium alginate and Sodium carboxymethyl cellulose in different concentrations. The resultant microspheres were evaluated for different in vitro and in-vivo evaluation parameters. In in-vitro studies, it was observed that increased concentration of Chitosan had a positive effect on the encapsulation of all drugs. The particle size increased with increasing of drug-polymer ratio. Bioadhesion was influenced with increase in pH and it was more rapid in intestine, rather than in stomach. The drug-polymer interaction was also studied by conducting FTIR and DSC tests. To assess the stability of all formulations for two years, the short term stability studies of the selected batches were carried out by keeping them at 40°C ± 2°C and 75% ± 5% RH for a period of six months.
Mucoadhesive microspheres, Metronidazole, In vitro release, Bio adhesion.
The most desirable and convenient method of drug administration is the oral route due to the ease of administration and patient compliance [1]. One limitation for oral delivery is poor bioavailability and for the drug candidates who show absorption window in the proximal guts and is the major obstacle to the development of controlled release formulation [2]. A number of approaches have been developed to increase the residence time of dug formulation. Microsphere carrier systems made from the naturally occurring biodegradable polymers have attracted considerable attention for several years in sustained drug delivery [3,4].
This can be achieved by coupling bioadhesion characteristics to microspheres and developing “Mucoadhesive microspheres”. Drug action can be improved by developing new drug delivery system, such as the mucoadhesive microsphere drug delivery system [5,6]. Metronidazole is anti-protozoa and anti-bacterial agent for treating amebiasis caused due to Entamoeba histolytica infection. In management of Entamoeba histolytica infection dose is 500 mg daily. The bioavailability is 80% and biological half-life is 8 hrs average of metronidazole for the oral administration, so can development of a sustained release formulation and reduce the dose frequency.In recent study we had tried to develop oral mucoadhesive drug delivery systems of metronidazole for an effective and safe treatment of Entamoeba histolytica. Polymers used for formulation are Chitosan, Sodium alginate, Hydroxy propyl methyl cellulose, Sodium carboxy methyl cellulose.
Metronidazole was obtained as a gift sample from orbit on pharma, Gujarat, India. Chitosan, Sodium alginate, HPMC K4 M, Sodium carboxy methyl cellulose were supplied by S.D. Fine Chemicals Ltd., Mumbai. All solvents used were of analytical grades and were used as obtained.
Formulation of microspheres
An accurately weighed quantity of metronidazole was added in increments to 100 ml of alginate solution to afford homogeneous dispersion. For preparation of modified alginate microspheres, chitosan of varying quantities were added to above prepared homogenous dispersion and mixed until homogeneous. In case of formulation containing sodium carboxy methylcellulose, it was equally mixed with alginate solution prior to drug addition. Each dispersion (20 ml) was dropped using syringe needle 16 G into slowly agitated 100 ml solution of 1.5% (w/v) calcium chloride. The resultant microspheres were washed once with distilled water and transferred into 100 ml of 1% w/v chitosan solution and 1% w/v acetic acid for 10 minutes [7] (Table 1).
| Formulation | Amount of sodium alginate (gm) | Amount of drug (gm) | Polymers | Amount of additive Polymer (gm) | Drug/Na.alginate ratio | Coagulation medium CaCl2 solution |
|---|---|---|---|---|---|---|
| MZ-1 | 2 | 5 | - | 0 | 2.5:1 | 1.50% |
| MZ-2 | 2 | 5 | Sodium CMC | 1 | 2.5:1 | 1.50% |
| MZ-3 | 2 | 5 | Sodium CMC | 2 | 2.5:1 | 1.50% |
| MZ-4 | 2 | 5 | Sodium CMC | 3 | 2.5:1 | 1.50% |
| MZ-5 | 2 | 5 | Chitosan | 1 | 2.5:1 | 1.50% |
| MZ-6 | 2 | 5 | Chitosan | 2 | 2.5:1 | 1.50% |
| MZ-7 | 2 | 5 | Chitosan | 3 | 2.5:1 | 1.50% |
| MZ-8 | 2 | 5 | HPMC K4M | 1 | 2.5:1 | 1.50% |
| MZ-9 | 2 | 5 | HPMC K4M | 2 | 2.5:1 | 1.50% |
Evaluation of microspheres
Particle size analysis and swelling studies: Size of the microspheres can be measured using micrometer. Studies related to swelling were needed to determine swelling behavior of multi particulate system in stomach and intestinal pH environment. Simple method of determining swelling capacity is observation of swelling of a formulation and nothing the increase in volume of formulation.
Entrapment efficiency: It can be determined by direct and indirect methods. In indirect methods, aliquots from filter solutions remaining after removal of microspheres were assayed for drug content. The amount of drug entrapped was calculated from the differences between the total amount of drug added and the amount of drug found in filtered solution.
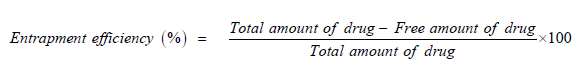
In indirect methods, accurately weighed quantities of microspheres were kept in a suitable buffer solution for sufficient time that breaks cross linked structure and liberates entrapped drug. Theoretical quantity of drug was calculated as a ratio of added drug amount to total amount of drug and additives [8].
The entrapment efficiency can be calculated by following formula:
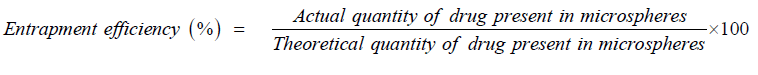
Determination of morphology by scanning electron microscopy
SEM is useful for characterizing the size and morphology of microscopic specimens in order to correlate other determined characteristics such as surface area and bulk density. SEM creates a magnified image using electrons instead of light and yields both topographic images and elemental information when used in conjunction with energy-dispersive x-ray analysis or wavelength-dispersive x-ray spectrometry. SEM produces a higher resolution compared to that possible using a light microscope, and the images obtained are three-dimensional. The maximum resolution for SEM (minimum distance by which the two objects can be separated and observed as distinct objects) is 10–20 nanometers compared to 200-300 nanometers for light microscopy [9].
Instrument used: JEOL SM6360 A, Datum Ltd, Tokyo, Japan
Pressure: 0.001 mm of Hg
Acceleration voltage: 20 KV.
In vitro wash-off test: The mucoadhesive properties of the microspheres was evaluated by in vitro wash-off test which is a simple and quick method as follows pieces of tissue (pig stomach about 2 x 5 cm and small intestine about 2 x 15 cm, obtained from slaughter house and stored in tyrodes solution) were tied onto a plastic slide (about 2 x 15 cm) using rubber bands. Microspheres were spread (25) onto each wet, rinsed tissue specimen, and counted. Immediately thereafter, the prepared two slides were connected with suitable support onto one of them groves of a USP tablet disintegrating test apparatus, permitting a slow, regular up and down movement (30 min-1) in a test fluid (0.1 N HCl, pH 1.2 and phosphate buffer pH 6.8) kept at 37°C. At given intervals, the motor was stopped and number of microspheres still adhering onto the tissue was counted. The results obtained can be used as a measure of bioadhesion [10,11].
The composition of tyrodes solution is sodium chloride 8 gm, potassium chloride 0.2 gm, calcium chloride 0.134 gm, sodium bicarbonate 1 gm, sodium dihydrogen phosphate 0.05 gm and glucose 1 gm per litre.
In vitro drug release studies: Drug release from prepared systems can be assessed by means of dissolution tests on formulations containing different amounts of chitosan and other systems and incorporation of model drugs that differ in their solubility’s in water at physiological pH levels.
Fourier Transform Infrared analysis (FT-IR)
Infrared spectroscopy is the subset of spectroscopy that deals with the infrared region of the electromagnetic spectrum. It covers a range of techniques, with the most common type by far being a form of absorption spectroscopy. As with all spectroscopic techniques it can be used to identify a compound and to investigate the composition of a sample without tiresome evaluation methods. FT-IR measurements of pure drug, polymer for ideal batch and of marketed preparations were obtained.
Instrument used: FT-IR spectrophotometer, 8400, Shimadzu,Japan.
Method adopted: Potassium bromide pellet (disc method).
Scanning range: 4500 to 350 cm-1 at the ambient temperature.
In-Vivo evaluation of selected formulations: The in vivo behavior of the gastro retentive bio adhesive microspheres of optimized formulation in the rabbit stomach was observed in real time using a radiographic imaging technique. Study is conducted on healthy male albino rabbits weighing 2-2.5 kg.
Preparation of placebo barium sulfate microspheres using composition of selected batch: The radio-opaque microspheres of optimized formulation of selected batch were prepared by the earlier mentioned method, replacing drug with sufficient quantity of Barium Sulfate. The other parameters of formulation were kept constant.
The in-vivo gastro-retention study was carried out by administering a placebo microspheres filled in hard gelatin capsule using a gastric feed tube to the overnight fasted rabbits (2-2.5 kg, n=3). After that, 10-12 ml water was given immediately. During the experiment, the rabbits were not allowed to eat any food, but water was available adlibitum. Then, the radiographic images were taken at various time intervals [12].
Stability studies
A stable product is one that shows no significant degradation or changes in its physical, chemical, microbiological, and biological properties, with the product remaining within its specification. The stability of pharmaceutical ingredients and the products containing them depends on (a) the chemical and physical properties of the materials concerned (including the excitements and container systems used for formulated products) and (b) environmental factors such as temperature, humidity, and light and their effect on the substances in the product. To evaluate the long-term stability (2 years), the selected formulations were stored at 40°C ± 2°C/75% ± 5% relative humidity (RH) for 6 months in a humidity chamber and were observed for physical change and drug content. At the end of storage period, the formulations were also subjected to drug release studies in intestinal fluids [13].
Particle size analysis
The size of microspheres is shown in Table 2. The length and breadth of the microspheres were measured. The length of the microspheres was between 0.734 ± 0.29 and 0781 ± 0.49 mm and whereas the breadth was between 0.74 ± 0.39 and 0768 ± 0.34 mm for all the metronidazole formulations. The size of microspheres increased with increasing amount of chitosan in the formulations. All the microspheres were intact after drying. The particle size of 25 microspheres of each metronidazole formulation was measured by digital screw gauge and the average values were calculated (Table 2).
| Particle size(mm) | ||
|---|---|---|
| Length | Breadth | |
| Formulations | Mean (±) SD | Mean (±) SD |
| MTZ-1 | 0.76 ± 0.38 | 0.75 ± 0.27 |
| MTZ-2 | 0.73 ± 0.83 | 0.73 ± 0.03 |
| MTZ-3 | 0.73 ± 0.93 | 0.72 ± 0.34 |
| MTZ-4 | 0.75 ± 0.17 | 0.73 ± 0.74 |
| MTZ-5 | 0.75 ± 0.39 | 0.73 ± 0.33 |
| MTZ-6 | 0.74 ± 0.43 | 0.74 ± 0.05 |
| MTZ-7 | 0.74 ± 0.29 | 0.71 ± 0.38 |
| MTZ-8 | 0.76 ± 0.53 | 0.73 ± 0.37 |
| MTZ-9 | 0.73 ± 0.05 | 0.74 ± 0.29 |
Entrapment efficiency
The drug in microspheres was determined by indirect method (digestion method) drug loaded microspheres (50 mg) were placed 500 ml conical flask containing 200 ml of 0.1 N HCl solution in water. The microspheres were stirred to release metronidazole completely. These solutions were centrifuged and filtered through a 0.45 μ m membrane filter. The filtrate was then sufficiently diluted with 0.1 N HCl solution. Aliquots of these solutions were subjected in triplicate to ultraviolet at 278 nm against 0.1 N HCl solutions as blank. The entrapment capacity was determined and results were shown in Table 3. Further it is observed that presence of anionic polymers sodium carboxy methyl cellulose causes only a slight decrease in entrapment efficiency of metronidazole compared to the formulation MTZ-1, but greater entrapment efficiency of metronidazole than that of formulations containing chitosan. In the metronidazole formulations MTZ-8 and MTZ-9 effect of concentration of calcium chloride solution on entrapment efficiency was not observed. This may be measure of the fact that higher amount of metronidazole was used for the preparation of all the formulations. The entrapment efficiency of metronidazole was between 64% and 96% on varying composition of the formulations. Formulations with more than 60% entrapment efficiency could be prepared under the chosen conditions (Table 3).
| Entrapment Efficiency | |
|---|---|
| Formulations | Mean (±) SD |
| MTZ-1 | 94.15 ± 0.36 |
| MTZ-2 | 74.60 ± 0.13 |
| MTZ-3 | 62.30 ± 0.41 |
| MTZ-4 | 51.43 ± 0.50 |
| MTZ-5 | 81.78 ± 0.27 |
| MTZ-6 | 73.20 ± 0.06 |
| MTZ-7 | 75.10 ± 0.55 |
| MTZ-8 | 87.20 ± 1.06 |
| MTZ-9 | 78.54 ± 0.67 |
Swelling properties of microspheres
The swelling behaviour of the all formulations was evaluated in 0.1 N HCl (pH 1.2) and phosphate buffer solution (pH 6.8). A representative fraction of the microspheres was used to study the swelling of the microspheres 10 ml graduated measuring cylinders were filled with 100 mg of microspheres and pH solutions (1.2 or 6.8) at 370°C were then added. The length of microsphere column was noted. The length of microsphere column and there by swelling was then measured at 3 h. The total volume of pH solution was constant throughout the experiment. These studies were done in triplicate and average results were calculated (Table 4).
| Relative swelling(at 3 h) | ||
|---|---|---|
| pH 1.2 | pH 6.8 | |
| Formulations | Mean (±) SD | Mean (±) SD |
| MTZ-1 | 17.60 ± 0.25 | 22.10 ± 0.24 |
| MTZ-2 | 16.20 ± 0.30 | 21.84 ± 0.10 |
| MTZ-3 | 15.52 ± 0.29 | 21.45 ± 0.18 |
| MTZ-4 | 23.10 ± 0.20 | 22.10 ± 0.72 |
| MTZ-5 | 16.10 ± 0.15 | 27.77 ± 0.13 |
| MTZ-6 | 21.50 ± 0.10 | 19.04 ± 0.26 |
| MTZ-7 | 25.18 ± 0.28 | 26.06 ± 0.10 |
| MTZ-8 | 22.10 ± 1.65 | 21.42 ± 0.07 |
| MTZ-9 | 23.10 ± 0.22 | 24.80 ± 0.30 |
In vitro wash-off test: This was done using 25 microspheres of each formulation by the methods described earlier i.e., wash-off test and falling liquid-film method. Bioadhesion tests were performed in triplicate for each experiment to calculate an average bioadhesion number. Results of in vitro bioadhesion testing are shown in Tables 5 and 6 and in Figures 1 and 2 respectively. Chitosan as outer layer in coated (MTZ-7) or double coated formulations showed good bioadhesion compared to coated formulation without chitosan (MTZ-9) and uncoated formulation. These results indicate that chitosan dispersed system or coated system have good mucoadhesion than plain alginate system (Tables 5 and 6, Figures 1 and 2).
| Mucoadhesion of microspheres Method-I | ||
|---|---|---|
| Formulations | Stomach | Intestine |
| Mean (±) SD | Mean (±) SD | |
| MTZ-1 | 46.00 ± 81 | 62.33 ± 14.43 |
| MTZ-2 | 82.57 ± 0.67 | 82.05 ± 0.57 |
| MTZ-3 | 81.67 ± 5.11 | 88.23 ± 7.22 |
| MTZ-4 | 83.10 ± 4.64 | 92.00 ± 4.76 |
| MTZ-5 | 33.40 ± 4.83 | 63.00 ± 4.4 |
| MTZ-6 | 78.57 ± 4.30 | 92.67 ± 6.11 |
| MTZ-7 | 90.00 ± 4.36 | 83.57 ± 6.15 |
| MTZ-8 | 15.67 ± 4.19 | 24.47 ± 9.25 |
| MTZ-9 | 62.23 ± 16.07 | 39.67 ± 9.10 |
| Mucoadhesion of microspheres Method-II | |
|---|---|
| Formulations | Intestine |
| Mean (±) SD | |
| MTZ-1 | 24.23 ± 10.2 |
| MTZ-2 | 86.7 ± 1.42 |
| MTZ-3 | 89.0 ± 4.1 |
| MTZ-4 | 88.0 ± 4.0 |
| MTZ-5 | 48.6 ± 5.1 |
| MTZ-6 | 87.3 ± 6.1 |
| MTZ-7 | 94.6 ± 7.1 |
| MTZ-8 | 28.3 ± 15.1 |
| MTZ-9 | 78.6 ± 15.2 |
Determination of morphology by scanning electron microscopy
The SEM of MTZ-3 and MTZ-6 were performed as depicted in Figure 3. The surface topography indicated that the microspheres were rough in appearance and completely covered with the polymers. The loading of the active drug did not cause any significant change in morphology. The inside of the microspheres was completely filled, indicating that complexation had occurred everywhere within the microspheres (Figure 3).
In-vitro drug release studies: The results of in vitro drug release studies were shown in Table 7 and Figures 4-6 respectively. The drug release profiles of the microspheres were determined using buffer media (pH 6.8). Metronidazole was released rapidly from formulations MTZ-1 and MTZ-5 and slowly from formulations MTZ-2, MTZ-3 and MTZ-4. Formulation MTZ-6, which dispersed as well as coating of chitosan, showed slow release of metronidazole, compared to formulation MTZ-7. Metronidazole release from formulation MTZ-8 was fast and whereas in coated formulations MTZ-9, it was slow. More than 20% of entrapped metronidazole was released from all the formulations at 30 minutes. More than 80% of incorporated metronidazole had been released at about 3.5 h from all the formulations (Table 7, Figures 4-6).
Fourier Transform Infrared analysis (FT-IR)
The FT-IR spectra of Chitosan, Metronidazole and MTZ-6 are shown in Figures respectively. The IR spectra of chitosan shows OHstretching for free OH group at 1309 cm-1, N-H stretching of primary amine at 3466 cm-1 and methylene group stretching at 2855 cm-1 and C=O bond of acetamide absorbed at 1665 cm-1. The IR spectra for Metronidazole shows C-NO2 nitro compounds aromatic at 1541.18 cm-1,while C-OH alcoholic hydroxyl group at 3286.81 cm-1,-CH3 at 2850.88 cm-1,-CH2 bending at 1487.17 cm-1 and C-N stretching vibrations at 1267.27 cm-1.The IR spectra for MTZ-6 shows C-NO2 nitro compound aromatic at 1498.88 cm-1, while C-OH alcoholic hydroxyl group at 3296.46 cm-1,-CH3 at 2858.60 cm-1,-CH2 bending at 1494.88 cm-1 and C-N stretching vibrations at 1273.06 cm-1. The IR spectrum of chitosan represents all the peaks of purity. Resemblance in the peaks for Metronidazole with the MTZ-6 formulation indicates compatibility with the polymers in the formulation. There was no significant difference in the peak characteristics which indicates the stability of the formulation.
In-vivo evaluation of selected formulations: The digital X-ray obtained for radio-opaque placebo microspheres in rabbits provide the evidence of bio adhesive nature of microspheres in rabbit's stomach. The BaSO4 tagged microspheres, similar to selected formulation was observed in stomach region till 12 ± 1.2 hour. The slight decrease in gastric retention compared to in vitro studies may be due to the presence of the peristaltic movement of the stomach during in vivo studies (Figure 7).
Stability studies
The stability studies were conducted for MTZ-3 and MTZ-6 batches, as they showed ideal properties such as encapsulation efficiency, sustained drug release and bio adhesion. When the dissolution study was conducted in the simulated intestinal fluids as described above, there was no significant difference (p<0:05) in the cumulative percent of metronidazole released from both MTZ-3 and MTZ-6 stored for 6 months when compared with that released from the same formulations before storage (Figure 8).
The current examination was done to develop oral mucoadhesive delivery system of metronidazole for a effective and safe treatment of Entamoeba histolytica infection. Changing the composition could make formulations of metronidazole with different drug release profiles and bio adhesive properties. Chitosan dispersed or coated formulations gradually released the metronidazole incorporated and showed good mucoadhesion to stomach and intestinal tissues. Finally, the ideal formulation (MTZ-6) from the results of in vitro evaluation, based on swelling behavior, mucoadhesion potential and drug release profile, were selected for in vivo studies in comparison with marketed products of these drugs.