Research - Der Pharmacia Lettre ( 2022) Volume 14, Issue 8
Received: 15-Aug-2022, Manuscript No. DPL-22-74824;
Editor assigned: 19-Aug-2022, Pre QC No. DPL-22-74824;
Reviewed: 03-Sep-2022, QC No. DPL-22-74824;
Revised: 10-Sep-2022, Manuscript No. DPL-22-74824;
Published:
17-Sep-2022
, DOI: 10.37532/dpl.2022.14.04
, Citations: Priya,Vishnu Development and In vitro Evaluation of Capecitabine Loaded Polymeric NanoParticles for Tumor Targeted Drug Delivery Systems . Der Pharm Lett 14 (2022): 4-10
,
Copyright: �© Pittu Vishnu Priya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The present study revealed that ionic gelation technique followed by sonication can be used as an effective tool for preparation of Capecitabine nanoparticles. The main is to delay the release of Capecitabine so that the dosing frequency can be reduced by which we may decrease the side effects and improve the patient compliance. Investigation of the preparation, characterization and in vitro delivery of the Capecitabine nanoparticles was carried out. The different formulations of with different concentration of drug-polymer and surfactant were examined and finalized. Encapsulation efficiency of nanoparticles ranged between 68%-81%. The prepared particles showed good drug-loading capacity. The in vitro release studies showed that after the initial burst, all of the drug-loaded batches provided a continuous and slow release of the drug. . Formulation (F-2) showed the highest encapsulation efficiency i.e., 81%. It was found that as the concentration of chitosan increased, the percentage of encapsulation efficiency was also increased.
Capecitabine, Chitosan, Sodium alginate, Poly vinyl alcohol, Solvent evaporation method, Franz diffusion cell.
For the past few decades, there has been a considerable research interest in the area of drug delivery using particulate delivery systems as carriers for small and large molecules. Particulate systems like nanoparticles have been used as a physical approach to alter and improve the Pharmacokinetic and pharmacodynamics properties of various types of drug molecules [1]. Nanotechnology is the science of the very small. It is the use and manipulation of matter at a tiny scale. At this size, atoms and molecules work differently, and provide a variety of surprising and interesting uses [2]. Nanotechnology and Nano science studies have emerged rapidly during the past years in a broad range of product domains. It provides opportunities for the development of materials, including those for medical applications, where conventional techniques may reach their limits [3]. Nanotechnology should not be viewed as a single technique that only affects specific areas. Although often referred to as the ‘tiny science’, nanotechnology does not simply mean very small structures and products [4]. The major goals in designing nanoparticles as a delivery system are to control particle size, surface properties and release of pharmacologically active agents so as to achieve the site-specific action of the drug at the rationale rate and dose. Polymeric nanoparticles offer some specific advantages over liposomes. For instance, they help to increase the stability of drugs/proteins and possess useful controlled release properties [5,6]. Nanoparticles are defined as particulate dispersions or solid particles with a size in the range of 10-1000 nm. The drug is dissolved, entrapped, encapsulated or attached to a nanoparticle matrix [7]. Depending upon the method of preparation, nanoparticles, Nano spheres or Nano capsules can be obtained. Nano capsules are systems in which the drug is confined to a cavity surrounded by a unique polymer membrane [8]. The main aim of this study is to achieve prolonged release of Capecitabine such that the dosing frequency of the drug can be reduced by which we may reduce the side effects and increase the patient compliance. By formulating Capecitabine as nanoparticles we can directly deliver the drug to the cancer cell and prevent the normal cells from the adverse effects of Capecitabine.Capecitabine is used to help treat patients with Dukes' C colon cancer (colon cancer that has spread to lymph nodes in the area close to the colon), after having surgery. This medicine is also used to treat metastatic colorectal cancer (cancer of the colon or rectum that has spread to other parts of the body) [9,10].
Materials
Capecitabine was collected as a gift sample from Hetero labs, Jadcherla, polymers and other excipients were purchased from AR Chemicals, Hyd.
Methodology
Compatibility studies: The drug-polymer compatibility was ascertained by subjecting the drug and homogenates of drug and polymer to Infrared spectrophotometric study.
Fourier Transform Infrared Spectroscopy (FTIR): Assessment of possible incompatibilities between an active drug substance and different excipients forms an important part of the preformulation stage during the development of a dosage form. The use of FT-IR technique allows pointing out the implication of the different functional groups of drug and excipients by analyzing the significant changes in the shape and position of the absorbance bands. In this method individual samples as well as the mixture of drug and excipients were ground mixed thoroughly with potassium bromide (1:100) for 3-5 mins in a mortar and compressed into disc by applying pressure of 5 tons for 5 mins in hydraulic press. The pellet was kept in the sample holder and scanned from 4000 cm-1 to 400 cm-1 in FT-IR spectrophotometer. Then the characteristics peaks were obtained of all sample as well as mixtures.
Method of preparation of capecitabine loaded nanoparticles: Nanoparticle’s formulations were prepared by solvent evaporation method. The various different amount of polymers was dissolved in solvent mixture of methanol (2 ml) and dichloromethane (8 ml) very slowly on a magnetic stirrer and Capecitabine (100 mg) was added to it and the contents were allowed to stand at room temperature for 30 to 45 minutes with occasional overtaxing to allow complete solubilisation of drug and polymer. This solution was poured into 5 ml of each different concentration aqueous polyvinyl cool solution. The resulting solution was homogenized by using high pressure homogenizer for 3 minutes to form o/w emulsion. This emulsion was immediately added drop wise to 125 ml of aqueous PVA solution. The contents were stirred for 6 hrs. At room temperature with a magnetic stirrer to evaporate organic volatile solvent, allowing the formation of a turbid Nano particulate suspension. The suspension was filtered through membrane filter. The filtrate was centrifuged (1000 rpm for 10 minutes) and supernatant was collected. Further, the ultra-centrifugation (35000 rpm for 1 hr) was carried for supernatants. Following ultracentrifugation, the pellet was washed and collected two times with deionized water to remove adsorbed drug and was suspended in deionized waterto prevent clumping on storage (Table 1).
| Ingredients | Batch no. | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Chitosan | 100 | 200 | - | - |
| Sodium alginate | - | - | 100 | 200 |
| Capecitabine (mg) | 100 | 100 | 100 | 100 |
Evaluation of capecitabine loaded nanoparticles
Particle size: All the prepared batches of nanoparticles were viewed under microscope to study their size. Size of liposomal vesicles from each batch was measured at different location on slide by taking a small drop of nanoparticle dispersion on it and average size of nanoparticles was determined.
SEM analysis: The morphology of NPs was studied by a scanning electron microscope. For this purpose, the sample was lyophilized and placed on aluminum stubs and the surface was coated with a layer of gold particles using a sputter coater. The shape of the NPs was determined by Scanning Electron Microscopy (SEM) (XL30, Philips, and The Netherlands) at 15 kV and 750 mA.
Drug encapsulation efficiency: Lyophilized nanoparticles 50 mg were dissolved in 100 ml of phosphate buffer and the drug amount was determined by UV analysis. The encapsulation efficiency was determined as the mass ratio of entrapped Capecitabine in nanoparticles to the theoretical amount of the drug used in the preparation. The entrapment of the Capecitabine nanoparticles was expressed as loading capacity.
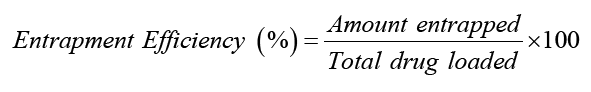
In vitro drug release studies: The release studies were carried out by Franz diffusion cell. It containing 10 ml phosphate buffer. Phosphate buffer pH 7.4 (100 ml) was placed in a 10 ml of beaker. The beaker was assembled on a magnetic stirrer and the medium was equilibrated at 37°C ± 50°C. Dialysis membrane was taken and one end of the membrane was sealed. After separation of non-entrapped Capecitabine dispersion was filled in the dialysis membrane and other end was closed. The dialysis membrane containing the sample was suspended in the medium. 1 ml of aliquots was withdrawn at specific intervals, filtered after withdrawal and the apparatus was immediately replenished with same quantity of fresh buffer medium. Time intervals: 1, 2, 3, 4, 5, 6, 7, 8 hrs.
Stability studies: Selected Formulation was subjected to stability studies as per ICH guidelines.
Following conditions were used for stability testing.
1. 25°C/60% RH analysed every month for period of one month.
2. 30°C/75% RH analysed every month for period of one month.
3. 40°C/75% RH analysed every month for period of one month.
In the present study 4 formulations with variable concentration of polymer were prepared and evaluated for physic-chemical parameters, in vitro release studies and stability studies.
Drug-excipient compatibility studies using (FTIR)
The compatibility between the drug and the selected lipid and other excipients was evaluated using FT-IR peak matching method. There was no appearance or disappearance of peaks in the drug-lipid mixture, which confirmed the absence of any chemical interaction between the drug, lipid and other chemicals (Figures 1 and 2).
Evaluation parameters
The nanoparticles prepared were evaluated as per the following parameters.
Particle size: The particle size increased with increasing surfactant concentration although the increase was not significant. Entrapment efficiency decreased with increasing PVA concentration. Based on particle size distribution and entrapment efficiency, PVA concentration was selected for further studies.
Surface morphology: Scanning Electron Microscopy (SEM) revealed that the MTX nanoparticles were smooth and spherical without any aggregation (Figure 3).
Drug entrapment efficiency: The first part of the plan of work was to optimize the concentration of polymers to be used in the formulation of nanoparticles. The optimization of polymer concentration was done on the basis of particle size and entrapment efficiency of nanoparticles obtained (Table 2).
| Batch No. | Particle size (nm) | Entrapment |
|---|---|---|
| Efficiency (%) | ||
| F1 | 256 | 72 |
| F2 | 348 | 81 |
| F3 | 212 | 69 |
| F4 | 248 | 78 |
In vitro drug release studies: Results indicate that the formulation showed initial burst release followed by sustained release of the drug for a prolonged period of time. The rapid initial release may be attributed to the fraction of cabecetabine on the surface of nanoparticles. The in vitro drug release results revealed that the prepared nanoparticles would be able to control drug release for extended period of time (Table 3) (Figure 4).
| Time (hrs) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 1 | 22.55 | 24.45 | 25.32 | 26.55 |
| 2 | 34.25 | 31.26 | 32.82 | 35.6 |
| 3 | 41.82 | 46.7 | 41.77 | 44.55 |
| 4 | 51.65 | 57.54 | 50.25 | 52.55 |
| 5 | 62.28 | 63.85 | 60.52 | 63.58 |
| 6 | 72.25 | 75.8 | 71.56 | 70.88 |
| 7 | 79.85 | 84.63 | 82.5 | 85.15 |
| 8 | 88.56 | 95.55 | 91.52 | 93.2 |
The in vitro diffusion studies were performed in pH 7.4 buffer using dialysis membrane for 8 hours. Initially the release of drug from all the three batches was found to be about 25%-30% in 8 hrs.
This was due to the release of adsorbed drug from the surface of nanoparticles. Later on a constant and slow drug release was observed for 8 hrs. F2 formulation which had drug polymer of chitosan and was decided to be the optimized formulation.
Stability studies: There was no significant change in physical and chemical properties of the nanoparticles. The formulation F-2 after 3 months. Parameters quantified at various time intervals were shown (Table 4).
| Formulation Code | Parameters | Initial | 1st Month | Limits as per Specifications |
|---|---|---|---|---|
| F-2 | 25Ë?C/60% RH | 95.55 | 95.41 | Not less than |
| % Release | 85% | |||
| F-2 | 30Ë?C/75% RH | 95.55 | 95.45 | Not less than |
| % Release | 85% | |||
| F-2 | 40Ë?C/75% RH | 95.55 | 95.5 | Not less than |
| % Release | 85% |
The present research proposed novel formulation Capecitabine nanoparticles for controlled release. Investigation of the preparation, characterization and in vitro release of the nanoparticles was carried out. The different formulations of with various ratios of drug-polymer and surfactant were evaluated and optimized. The method used for the formulation of Capecitabine containing chitosan and sodium alginate nanoparticles was ionic gelation method followed by sonication to reduce the particle size.Nanoparticle’s formulations showed good results in terms of the assayed drug content and encapsulation efficiency. This indicates that the method used for the formulation produced good yield and it was suitable and reproducible in nature. Formulation (F-2) showed the highest encapsulation efficiency i.e., 81%. It was found that as the concentration of chitosan increased, the percentage of encapsulation efficiency was also increased. Permeation studies with dialysis membrane were carried out as per the method reported. The formulations showed good drug release from the polymer, the in vitro drug release profiles of all the formulations showed an initial burst effect, and followed by a slow drug release. The burst release of drug is associated with those drug molecules dispersing close to the nanoparticle surface, which easily diffuse in the initial incubation time. The Capecitabine release was faster for those nanoparticles with higher drug content.
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google Scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
Citation: Priya,Vishnu Development and In vitro Evaluation of Capecitabine Loaded Polymeric NanoParticles for Tumor Targeted Drug Delivery Systems . Der Pharm Lett 14 (2022): 4-10
Copyright: �© Pittu Vishnu Priya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.