Research Article - Journal of Natural Product and Plant Resources ( 2017) Volume 7, Issue 2
Studies on isolation and identification of fungi associated with banana rots were carried out. The effect of aqueous and ethanol extract of Citrus paradisi were determined in vitro on causative agents of post-harvest rot of banana. Concentrations of 20, 40 and 60% of both aqueous and ethanol extract of Citrus paradisi were used. The treatments were laid out in Completely Randomized Design (CRD) with three replications. Rhizoctonia solani, Rhizopus stolonifer, Colletotrichum musae and Pyricularia grisea were isolated and identified to be associated with the banana rots. Rhizoctonia solani has the highest frequency of occurrence with 37.50% followed by Rhizopus stolonifer with 26.25% and the least was Pyricularia grisea with 17.50%. Concentrations of both aqueous and ethanol extract (20, 40 60%), significantly (p< 0.05) inhibited radial mycelial growth of the fungi compared with the control. All citric extracts at varying concentrations were effective in reducing the mycelial growth and the effect was proportional to the concentration of the extract. The inhibition was highest at 60% concentration and lowest at 20% concentration. Both Aqueous and ethanol extract suppressed the growth of all the fungal isolates, but the ethanolic extract showed higher inhibition on fungal isolates. The study revealed that both aqueous and ethanol extract have antifungal properties to control banana rot caused by Rhizoctonia solani, Rhizopus stolonifer, Colletotrichum musae and Pyricularia grisea which serves as good option to synthetic fungicide which are hazardous and often costly.
Antifungal activity, Musa acuminata, Citrus paradisi, Aqueous and Ethanol extract, Jalingo.
Natural plant protectant with antimicrobial and antifungal properties have been advocated as an alternative to synthetic fungicides in the control of fungi associated with post-harvest rots of most vegetables and edible fruits, especially banana fruits (Musa spp). Banana being a highly perishable fruit suffers severe post-harvest losses both in terms of quality and quantity [1], and the major limiting diseases of most bananas in the world are phytopathogenic fungi, bacteria, and viruses. For example, 50 to 90% losses of marketable fruit have been reported from virus infection [2]. Bacterial spot caused by a seed-borne bacterial pathogen (Xanthomonas campestris pv. vesicatoria) is also capable of causing severe defoliation of plants, resulting in reduced yield and loss of quality of harvested fruit when severe damage occurs on enlarging fruits [3].
Medicinal plant materials have been successfully used for the treatments of fungal and bacterial infections in humans [4], suggesting that some plant materials may also possess antifungal and antibacterial constituents that are useful in controlling plant diseases [5]. Extracts of many higher plants have been reported to exhibit antibacterial, antifungal and insecticidal properties under laboratory trails [6-8].
Grapefruit extract for example contain different classes of polyphenolic flavonoids that have been shown to exert antifungal activities [9]. Citric extract of grapefruit is a liquid extract derived from the seeds, pulp, and white membranes of grapefruit [10]. This extract has been stated by some practitioners of alternative medicine to possess antibacterial, antiviral, and antifungal properties [11]. Previous report by [12] and [13] shows that grapefruit seed and pulp extract prepared or contaminated with ethanol, known as ethyl alcohol, is effective in inhibiting bacteria.
Application of synthetic fungicides is the most common practice for commercial control of banana rots [14, 15]. Although synthetic fungicides proved effective in the control of major postharvest diseases, their application may be harmful to human health and the environment and they become ineffective after prolonged use. Attempts have been made towards banana rot control through cultural, physical and biological [15] methods as an alternative to synthetic fungicides [16].
As researchers are looking for alternative to these agrochemicals [17] natural plant products derived from plants effectively meet this criterion and have enormous potential to influence modern agrochemical research [18]. The use of natural plant pesticides is now emerging as one of the prime means to protect crops and their products and the environment from pesticides [19]. Plant pesticides degrade more rapidly than most chemical pesticides, and therefore are considered to be ecofriendly and less likely to kill beneficial pests than synthetic pesticides with longer environmental retention [18]. Most of the plant pesticides generally degrade within a few days, and sometimes even within a few hours [20].
Despite the alarming rate of spoilage caused by fungi on banana fruit, little or no researched has been carried out to test the fungi toxic properties of these plants pesticides in the study area which serves as an alternative to synthetic fungicide to small scale farmers and therefore, the objective of the study was to isolate and identify fungi causing post-harvest rot of banana fruit. In addition, test on the efficacy of aqueous and ethanol extract of grapefruit on the isolated organism was also evaluated.
Study Area
The study was conducted in Biological Science Laboratory, Taraba State University, Jalingo. Taraba State. The University is located between latitudes 8°47′ to 9°01′N of the equator and longitudes 11°09′ to 11°30′ E of the Greenwich Meridian. The city of Jalingo within which the University is located in the administrative headquarters of Taraba State with climatic condition typical of the tropics having raining and dry seasons. The raining seasons starts from May to October while the dry season commences from November to April. It has an annual mean rainfall of 125mm and temperature of 30°C to 40°C [21].
Collection of banana fruits
Banana fruits both healthy and diseased were collected from Jalingo Main Market located in Jalingo, Taraba State, Nigeria. Samples of Musa acuminata (L) fruit showing spoilage and rotting were collected from different selling points randomly in the market. Both the fresh and the diseased banana fruits of uniform sizes were packed into sterilized polythene bags and taken to the Biological Science Laboratory at Taraba State University (TSU), Jalingo for isolation, pathogenicity test and control trials.
Isolation and Identification of Fungi from banana fruit
The banana fruits showing deterioration and rot symptoms were aseptically cut into sections of approximately 5mm square with a heat sterilize knife. The sectioned fruits were surface sterilized with 1% sodium hypochlorite for 30 seconds and rinsed with three (3) changes of sterile distilled water to remove surface contaminants. The sterilized sectioned fruits were dried between filter papers and then plated on 9cm diameter already prepared PDA media. The plates were incubated at room temperature of 25 ± 20C for 3 days before sub-culturing on fresh sterile PDA plates. The sub-culturing on fresh sterile media was carried out adopting the method of [22] until pure cultures were obtained.
Identification of the isolated fungi was carried out based on their cultural characteristics on growing media. Hyphae containing spores were picked using a sterile needle and placed on a sterile glass slide with a few drops of Lactophenol cotton blue and were examined under photographic microscope using the method of [23]. The cultural and morphological features observed were compared with structures in the identification guides of the International Mycological Institute Kew and [24].
Determination of the frequency of occurrence (FC) of the isolated Fungi
The frequency of occurrence of the isolated fungi was determined by using the percentage incidence of occurrence as follows:
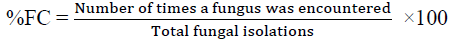
Pathogenicity Test
Healthy banana fruit (semi ripe) were surfaced sterilized with 0.5% sodium hypochlorite for 30 seconds and rinsed in three changes of sterile distilled water adopting the approach of [22]. A 5 mm diameter flame-sterilized cork borer was used to puncture and remove a cylindrical core from the healthy. The healthy fruits were then aseptically inoculated with seven (7) day old fungal culture of the isolates in three replicates. The cylindrical core removed was then replaced and Vaseline jelly was smeared to completely seal the hole to prevent external infection or contamination. It was then incubated at room temperature of 25±20C. The control was inoculated in the same manner except that sterile agar was used instead of the isolates. For each fungal isolates, three (3) banana fruit (Musa acuminata L.) were inoculated and three controls were also set up. All experiment was carried out in the laboratory using completely randomized design and was incubated for seven (7) days. Symptoms of rots observed from different fungal isolates were compared to the original natural rot.
Collection and extraction of plant materials
Plant materials used were seed and juiceless pulp of Citrus paradisi. The seed and juiceless pulp of the grape fruit were collected, washed and air-dried for two weeks to prevent loss of active component. Then were ground into powder with electric blender, water and ethanol were used for extraction [25].
Forty grams of dry seed and juiceless pulp powder were placed in 160ml of sterile distilled water and left to stand at room temperature for 24hrs. The mixture was filtered with 3 layer cheese cloths and filtrate extract of 60%, 40% and 20% dilution was prepared using distilled water.
Ethanol extraction
The same method was used for the ethanol extract. Except that in this, the extract was placed into a wide tray to evaporate ethanol and equal quantities of distilled water were added to make citric extract.
Effect of citric extract on fungal Mycelial growth
The approach of [26] and [27] was used to evaluate the allelopathic effect of the citric extract on fungal growth by creating four equal sections on each plate by drawing two perpendicular lines at the bottom of the plate. The point of intersection indicated the center of the plates. This was done before dispensing PDA into each of the plates. About 60ml, 40ml and 20ml of the citric extracts of Citrus paradisi was separately introduced into the conical flask containing the same quantity of media (250mls). The amended media were plugged with cotton wool and heated for about 10 minutes to avoid contamination [28] before dispensing 10ml each into the Petri-dishes (poisoned food method) [29]. Each Petri dish was inoculated with 5 mm plug of pure isolate taken from margins of actively growing culture of pathogen. PDA plates free of the extracts were also prepared as control. Three (3) plates were used as replicates for each particular treatment as well as control. Then Petri plates were incubated at 25° ± 2°C until fungal growth in the control filled the whole petri dish, and then all treatments were examined.
Mean radial mycelial growth of each isolate was recorded and data were transformed into inhibition percentage by using the following formula [30].
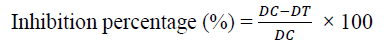
Where DC - Average Diameter of fungal spores germinated in control
DT - Average diameter of fungal spores germinated with treatment
Experimental Design and Statistical Analysis
Results and data collected were analyzed statistically using Statistical Analysis System version 7 and means that were significantly different were separated using protected Fisher’s Least Significance Difference test (LSD) at p < 0.05.
The isolated fungi from the banana fruit were Colletotrichum musae, Pyricularia grisea, Rhizoctonia solani and Rhizopus stolonifer. R. solani occurred more frequently with 37.50%, followed by R. stolonifer with 26.25%, Colletotrichum musae with 18.75% and the least was Pyricularia grisea with 17.50% (Table 1). All isolated fungi were pathogenic on banana fruits, from physical observation the symptoms were similar to those exhibited by the rotten banana fruits from which the organism were isolated. Rhizoctonia solani and Rhizopus stolonifer completely disintegrated the affected tissue (100% infection) with extensive rots covering the fruit and mycelial growth on PDA within 3-4 days. The rot induced by Colletotrichum musae and Pyricularia grisea shows moderate to severe infection.
| Fungi isolated | Numbers of times isolated | Percentage frequency (%) |
|---|---|---|
| P.grisea | 14 | 17.50% |
| R.solani | 30 | 37.50% |
| R.stolonifer | 21 | 26.25% |
| C.musae | 15 | 18.75% |
Table 1: Percentage frequency of occurrence of fungi from rotted banana fruits.
All concentration of aqueous citric extract significantly (p < 0.05) suppressed the mycelial growth of the four tested pathogens (Table 2), the effect was proportional to the concentration of the extract and this was also statistically significant (p < 0.05). The inhibition was highest at 60% concentration and lowest at 20% concentration. However, aqueous extract inhibited the growth of Colletotrichum musae more than R.solani, R.stolonifer, and Pyricularia grisea.
| Concentration (%) | Inhibition (%)Â Fungal pathogens | |||
|---|---|---|---|---|
| P. grisea | R.solani | R.stolonifer | C.musae | |
| 20 | 33.55 | 1.25 | 7.08 | 60.85 |
| 40 | 42.27 | 6.58 | 9.32 | 79.99 |
| 60 | 51.66 | 13.9 | 15.29 | 85.45 |
| Control | 0 | 0 | 0 | 0 |
| Mean | 31.87 | 5.43 | 7.93 | 56.57 |
| LSD (p < 0.05) | 6.37 | 4.74 | 3.54 | 9.25 |
Table 2: Inhibition effect of aqueous extract of citrus paradisi on the mycelial growth of pathogen.
All concentration of ethanol extract also significantly (p < 0.05) suppressed the mycelial growth of the isolated organism and the effect was also proportional to the concentration of the extract. The inhibition was highest at 60% concentration and lowest at 20%. However, the toxicity of ethanol extract was higher than that of aqueous extract in the percentage growth inhibition of the four tested pathogens (Table 3).
| Cocentration (%) | Inhibition (%) | |||
|---|---|---|---|---|
| P. grisea | R.solani | R.stolonifer | C.musae | |
| 20 | 51.91 | 40.51 | 9.25 | 37.64 |
| 40 | 84.6 | 59.83 | 87.4 | 85.1 |
| 60 | 85.57 | 93.97 | 94.07 | 85.03 |
| Control | 0 | 0 | 0 | 0 |
| Mean | 55.52 | 48.58 | 47.68 | 60.29 |
| LSD (p < 0.05) | 13.98 | 4.26 | 4.6 | 3.21 |
Table 3: Inhibition effect of ethanol extract of citrus paradisi on the mycelial growth of pathogen
The study showed that a number of fungi are associated with post-harvest rot diseases of banana fruits in the study area. These fungi include Pyricularia grisea, Rhizoctonia solani, Colletotrichum musae and Rhizopus stolonifer. The result of the pathogenicity test carried out showed that the fungal organisms re-isolated had the same characteristics with those isolated originally from the rotten banana fruits; hence they are the causal agent of the fruits rot observed. This agrees with the report of [31, 32, 33, 34, 35] that Pyricularia grisea, Rhizoctonia solani, Colletotrichum musae and Rhizopus stolonifer were among the fungi associated with the post-harvest fruits rot of banana (Musa spp).
All the fungal isolates are pathogenic on the banana fruits used in the study, although they differ to some extent. The results further revealed that Rhizoctonia solani is the most pathogenic owing to the size of rot caused and this was in agreement with the findings of [35] who reported that Rhizoctonia solani was responsible for the fruit spoilage of banana. [36] also reported on the pathogenic effect of Rhizopus stolonifer on Daucus spp and Carica papaya. Colletotrichum musae and Pyricularia grisea shows moderate to severe effect on the banana fruits.
The aqueous and ethanol extract of Citrus paradisi tested for antifungal activity against four fungi isolated from banana fruits showed that both aqueous and ethanol extract tested showed antifungal activity against all organism isolated. The antifungal activity of citric extracts was reported by [11], [37] who studied extracts of citric seeds under the post-harvest period. All concentration of aqueous extract of Citrus paradisi suppressed the mycelial growth of the four tested pathogens, the effect was proportional to the concentration and inhibition value at 60% (higher concentarion), was higher for Colletotrichum musae followed by Pyricularia grisea. However, lowest inhibition value was observed from Rhizoctonia solani. All concentration of ethanol extract of Citrus paradisi suppressed the mycelial growth of the four tested pathogens, and this was also reported by [38] who noticed that ethanol extracts of A.indica showed fungitoxic properties against 5 pathogenic fungi (Alternaria brassicola, Colletotrichum capsici, F.oxysporum, R.solani and Sclerotinia sclerotiorum) when tested under laboratory conditions at 500 and 1000 μg/ml. Previous report by [12, 13] shows that grapefruit seed and pulp extract prepared or contaminated with ethanol, known as ethyl alcohol, is effective in inhibiting bacteria.
The effect of ethanol extract of Citrus paradisi was also proportional to the concentration and inhibition value at 60% concentration (highest concentration), was higher for Rhizopus stolonifer, followed by Rhizoctonia solani and the lowest inhibition value was observed from Colletotrichum musae. Comparing Citric extracts of aqueous with ethanol, the inhibition percentage in each increased gradually with the extract concentration, both suppressed the growth of all the fungal isolates but the ethanolic extract showed higher inhibition on the growth of all the fungal isolates. Further investigation is therefore suggested on testing the fungicidal effect of the extract in-vivo on the fruit and to fractionate the extract to identify, isolate, purify and then characterize the principles responsible for the reported control. The study shows that the used of aqueous and ethanol citric extract against banana rot pathogens would have better result as they are biologically based and environmentally safe.
The findings from this study revealed that most rots of banana in Yola are caused by these four isolated fungi; they are Colletotrichum musae, Pyricularia grisea, Rhizoctonia solani and Rhizopus stolonifer. All were found to be highly pathogenic on banana fruit. The inhibitory effect of the citric extracts against fungal isolates shows that the extract proved effective in the control of all the pathogens and this could be due to the presence of antifungal substances present in the extract. Higher inhibition of fungal growth was observed at higher concentrations of the aqueous and ethanol extracts. The result also indicated that ethanol is better solvent than water for the extraction of the active ingredients of the extract.
The author wish to acknowledge the contribution and support of Dr. F.K. Channya, Prof. I.B. Chimbekujwo and Dr. Delphine.L.David for their assistance towards the success of this work.