Research Article - Der Pharmacia Lettre ( 2025) Volume 17, Issue 1
Received: 11-Aug-2023, Manuscript No. DPL-23-110171;
Editor assigned: 15-Aug-2023, Pre QC No. DPL-23-110171 (PQ);
Reviewed: 29-Aug-2023, QC No. DPL-23-110171;
Revised: 18-Jan-2025, Manuscript No. DPL-23-110171 (R);
Published:
25-Jan-2025
, DOI: 10.37532/0975-5071.2025.17.01
, Citations: Gupta S, et al. 2025. Synthesis and Evaluation for Anti-Infective Activities of 2-Arylidene-1-(4-Methyl-6-
Phenylpyrimidin-2-YL) Hydrazides. Der Pharma Lett.17:01-13.
,
Copyright: © 2025 Gupta S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Hydrazones constitute chemical entities, which are a vivid area of pharmaceutical, and drug candidates and spacious impedance as outstanding precursors for the discovery of numerous heterocyclic compounds. In addition, hydrazones pharmacophores are associated with profound biological activities, such as antimicrobial, antifungal, anthelmintic and antimalarial activity. The biological significance of benzaldehyde along with the pharmacological importance of hydrazine motivated us to synthesize some new benzaldehyde derivatives using pyrimidine as a starting material. In the present investigation, we describe the synthesis and anthelmintic, antibacterial, antifungal, and antimalarial evaluation of novel derivatives of selected drugs. The target compounds were synthesized taking pyrimidine as a starting material the yields of different synthesized compounds were found to be under different ranges and the structures of all the newly synthesized compounds were confirmed by suitable spectroscopic methods such as IR, and 1H NMR spectral data.
IR: Infrared Spectroscopy; NMR: Nuclear Magnetic Resonance Spectroscopy; 1H: Proton NMR; áµ?: Chemical shift; DMSO: Dimethyl Sulfoxide; %: Percent; μ: Mu (micro); RF: Retardation Factor; TLC: Thin-Layer Chromatography; IUPAC: International Union of Pure and Applied Chemistry
A very large number of groups of organic compounds are encountered with heterocyclics. They play a pivotal role in the metabolism of all cells, which are very diffuse and necessary for life. Mechanistic investigations enhanced the understanding of these compounds. Heterocyclic compounds have interesting theoretical implications, a diversity of synthetic procedures, and physiological and industrial significance [1].
Hydrazones are characterized by the grouping: C=N-N. They are distinguished from other members of this class (imines, oximes, etc.) by the presence of the two interlinked nitrogen atoms.
Hydrazones constitute chemical entities, which are the vivid area of pharmaceutical, and drug candidates and spacious impedance as an outstanding precursor for the discovery of numerous heterocyclic scaffolds. In addition, hydrazones pharmacophores are associated with profound biological activities, such as anticancer, anti-inflammatory, antifungal, insecticidal, antioxidant, and antitubercular. Acylhydrazone derivatives can exist in four forms with the collective effect of configurational stereochemistry E and Z as well as conformational stereoisomers or rotamers i.e., antiperiplaner (ap) and synperiplaner (sp) conformers (Figure 1) [2].
Figure 1: Structure of the hydrazone functional group.
Hydrazones are a major class of compounds for the development of new medicines that seek to find an effective compound in tackling multidrug-resistant bacterial infection. Hydrazones represent a resourceful compound of an organic class having the basic structure R1R2C=NNR3R4. Hydrazones and their derivatives are known to exhibit interesting diverse biological activities like anti-HIV, anti-tubercular anti-oxidant, anti-inflammatory, anti-microbial, anti-convulsant, analgesic, anti-cancer, anti-protozoal, anti-parasitic, and cardio-protective (Figure 2) [2].
Figure 2: Benzaldehyde phenyl hydrazone.
Hydrazones are usually named after the carbonyl compounds from which they are derived; thus benzaldehyde and phenylhydrazine give benzaldehyde phenylhydrazone (Figure 3).
Pyrimidines
Figure 3: Pyrimidine ring.
Pyrimidines as an important constituent of nucleic acids, pyrimidines have been occupying a distinct and unique place in human life and consequently in organic and medicinal chemistry research. Although, the moiety is mainly known for its biological significance, its importance in the field of material chemistry can never be underestimated as lots of pyrimidine derivatives are reported recently as a constituent of liquid crystal, fluorescence material, photoresponsive surfaces, etc. attracting the attention of material chemists also. Due to the immense biological, medicinal as well as material significance of pyrimidine derivatives, synthetic chemists have been paying more attention to the development of a novel, efficient methodology for various structurally important pyrimidine scaffolds which encourages us to carry out this piece of work [3].
The introduction of an additional ring to the pyrimidine core tends to exert a profound influence in conferring novel biological activities in these molecules. Pyrimidine and its fused derivatives play an essential role in several biological processes and have considerable chemical and pharmacological importance. A wide range of antibacterial and antistatic agents, agrochemicals, and animal health products can be characterized by the presence of pyrimidine nuclei. Thus, pyrimidine is capable of being used as an electron-withdrawing component in push-pull molecules which are highly deficient aromatic heterocycles. Furthermore, the luminescence ability may be triggered by transfers of intra-molecular charge along a molecule's backbone. Recent reports exhibit that pyrimidine derivatives are a good nonlinear material [4].
Biological evaluation
Anthelmintic evaluation: Anthelmintics are therapeutic agents used to destroy parasitic worms or to remove them from an infected host. The ultimate test of anthelmintic activity is the ability of a chemical agent to eliminate worms. The anthelmintic activity was evaluated on the adult Indian earthworm Pheretima posthuma due to its anatomical resemblance with the intestinal roundworm parasites of human beings. In the present work, the anthelmintic activity of newly synthesized compounds was evaluated using a variety of earthworms. Earthworms (Pheretima posthuma) were procured from an agriculture farm, Kurukshetra.
Suspension of the samples was prepared by triturating the samples with 0.2% of saline solution (salt in distilled water). The resulting mixture was then stirred with a mechanical stirrer for 30 min. The resulting suspensions were diluted to contain 10 mg of the test samples. These suspensions were used for anthelmintic activity.
The standard drug albendazole was also used in the suspension from the same concentration in a similar way. Suspension of distilled water and 0.2% DMSO were used as control.
Method: Anthelmintic activity was carried out by Garg’s method (Table 1) [6].
| S.no. | Sample | Concentration (20 ml) | Paralysis time ± SD | Death time ± SD |
|---|---|---|---|---|
| 1 | 6a | 0.20% | 29.8 ± 15.42 | 44.2 ± 43.82 |
| 2 | 6b | 0.20% | 17.4 ± 15.6 | 27.6 ± 26.96 |
| 3 | 6c | 0.20% | 6.2 ± 6.1 | 16.8 ± 4.96 |
| 4 | 6d | 0.20% | 72.4 ± 71.8 | 26 ± 25.7 |
| 5 | 6e | 0.20% | 6.4 ± 5.8 | 12.4 ± 12 |
| 6 | 6f | 0.20% | 19.8 ± 19.2 | 15.4 ± 14.93 |
| 7 | 6g | 0.20% | 9.8 ± 9.2 | 15.4 ± 14.93 |
| 8 | 6h | 0.20% | 30.8 ± 30.4 | 38.4 ± 37.5 |
| 9 | 6i | 0.20% | 87 ± 86.31 | 42.4 ± 41.83 |
| 10 | 6j | 0.20% | 71. 2 ± 55.16 | 17.8 ± 17.30 |
| 11 | Standard (Albendazole) | 0.20% | 14.4 ± 13.96 | 27.4 ± 26.92 |
Table 1: Anthelmintic activity of synthesized compounds.
Antimicrobial evaluation: Earlier studies have shown that substituted benzaldehyde derivatives possess antimicrobial activity. Given the above-mentioned work, an effort was made to check the antimicrobial and antifungal activities of the synthesized compounds [7].
The antibacterial action of a compound is said to be inhibitory when the normal multiplication rate of a bacterial culture is depressed, bacteriostatic when no multiplication occurs, and bactericidal when the number of viable bacteria is decreased. Under ordinary conditions, it is usually difficult to differentiate clearly between the three types of actions because all three may be effective simultaneously [8].
In our present study, all the synthesized derivatives of benzaldehydes have been evaluated for their antibacterial and antifungal activities by the agar well diffusion method.
Anti-bacterial evaluation: Antibacterial drugs exist from bacteria or molds or they are synthesized de novo. “Antibiotic" solely relates to antimicrobials made from bacteria or molds, yet it's frequently used in conjunction with antibacterial medication. There are numerous ways that antibiotics work, including by preventing the production of cell walls, activating enzymes that break down cell walls, increasing cell membrane permeability, and obstructing protein synthesis and nucleic acid metabolism. [9].
In-vitro methods
Turbidimetric method: This method depends on the growth of a microbial culture in a uniform culture of antibiotics in a fluid medium that is favorable to its rapid growth in the absence of antibiotics. Varying concentration of the compounds is added to test organism on liquid culture and turbidity produced is measured by taking absorbance and compared with turbidity produced by the standard drug [10].
Cup plate techniques: This technique relies on the diffusion of an antibiotic from a vertical cylinder or cavity through the hardening agar layers of a Petri plate to the extent that the growth of additional microorganisms is completely prevented in a circle surrounding the cylinder or cavity containing the antibiotic solution or the test compounds. Measured is the diameter of the clear zone created as a result of stopping microbial development [10].
Disk diffusion method: This method relies on the diffusion of an antibiotic from an antibiotic-impregnated disk through the solidifying agar layer on a plate to the extent that the growth of additional microorganisms is completely prevented in a circular area around the disk impregnated with the antibiotic or the test compounds. The zone of inhibition so produced is measured [10].
Experimental protocols
The activity was evaluated against four bacterial cultures namely
• Escherichia coli: Gram-negative
• Pseudomonas aeruginosa: Gram-negative
• Staphylococcus aureus: Gram-positive
• Streptococcus pyogenes: Gram-positive
The antifungal activity was evaluated against fungus namely:
Synthesized compounds 6a-6j were screened for anti-bacterial activities using the disk diffusion method and by Minimum Inhibitory Concentration (MIC) method.
Antifungal activity
Fungi are different from both viruses and bacteria. They are larger than bacteria and viruses. Fungi are heterotrophs i.e., Then secrete digestive enzymes and absorb the resulting soluble nutrients from whatever they are growing on. For this reason, they are often considered great decomposers in the ecosystem but can cause a problem when they begin to absorb nutrients from living organisms. They are most commonly breathed in or have contact with the skin. In favorable conditions, they begin to reproduce and cause diseases. Some antifungal agents are available to treat these infections. But it has been much more difficult for us to create successful antifungal drugs than antibacterial drugs because the fungal cellular structure resembles more closely to animal cells than bacterial cells. Thus, it remains a typical concept to develop agents that could selectively destroy the fungal cells without affecting the animal cells. The most successful drugs that have been created prevent the formation of chitin, therefore, prevent the fungus from creating new cell walls and spreading [11].
Test strains
For the present work efficacy of the test compound, it was determined against enzymes. The fungal cultures were sub-cultured in agar medium and incubated under the aerobic condition at 25°C for 48 hrs. This culture was used for the test [12].
Procedure
Each Petri plate containing solidified agar medium was inoculated with one fungal culture by spreading the suspension of the culture with a sterile cotton swab. Along the diameter, each plate was split into four equal pieces. One disk was positioned in each section. All plates were in the refrigerator for 30 min. to allow the diffusion of the sample into the surrounding agar medium. Then the plates were incubated at 25°C for 48 hours, and the results were tabulated. The diameter of the zones of inhibition wherever produced was measured and the average diameter of each sample was calculated. The results are given in the Table 2. The diameter was compared with that produced by the standard antifungal agent (Figure 4 and Table 3) [11-14].
| Minimum inhibitory concentration (microgram/ml) | |||||
|---|---|---|---|---|---|
| Sr.no | Compound no. | E. coli (MTCC 442) | P. aeruginosa (MTCC 441) | S. aureus (MTCC 96) | S. pyogenus (MTCC 443) |
| 1 | 6a | 200 | 250 | 100 | 125 |
| 2 | 6b | 100 | 250 | 100 | 200 |
| 3 | 6c | 125 | 200 | 200 | 100 |
| 4 | 6d | 100 | 100 | 125 | 100 |
| 5 | 6e | 200 | 125 | 100 | 250 |
| 6 | 6f | 50 | 100 | 100 | 100 |
| 7 | 6g | 62.5 | 100 | 125 | 100 |
| 8 | 6h | 100 | 200 | 250 | 100 |
| 9 | 6i | 100 | 100 | 100 | 125 |
| 10 | 6j | 62.5 | 100 | 100 | 100 |
| 11 | Std.1 | 0.05 | 1 | 0.25 | 0.5 |
| 12 | Std.2 | 100 | 100 | 250 | 100 |
| 13 | Std.3 | 50 | 50 | 50 | 50 |
| 14 | Std.4 | 25 | 25 | 50 | 50 |
| 15 | Std.5 | 10 | 10 | 10 | 10 |
| Standard 1: Gentamycin; Standard 2: Ampicillin; Standard 3: Chloramphenicol; Standard 4: Ciprofloxacin; Standard 5: Norfloxacin | |||||
Table 2: Antibacterial activity of inhibitory synthesized compounds.
Figure 4: Antimicrobial testing by disk diffusion test.
| Minimum fungicidal concentration (microgram/ml) | ||||
|---|---|---|---|---|
| Sr.No. | CompoundNo. | C. albicans (MTCC 227) | A. niger (MTCC 282) | A. clavatus (MTCC 1323) |
| 1 | 6a | 1000 | 1000 | 1000 |
| 2 | 6b | 500 | 1000 | 1000 |
| 3 | 6c | 250 | 1000 | 1000 |
| 4 | 6d | 1000 | 500 | 500 |
| 5 | 6e | >1000 | 500 | 500 |
| 6 | 6f | 250 | 500 | 1000 |
| 7 | 6g | 250 | 500 | 500 |
| 8 | 6h | 500 | 500 | 500 |
| 9 | 6i | 1000 | >1000 | >1000 |
| 10 | 6j | >1000 | 500 | 500 |
| 11 | Std. 1 | 100 | 100 | 100 |
| 12 | Std. 2 | 500 | 500 | 100 |
| Note: Standard drug 1: Nystatin; Standard drug 2: Greseofulvin | ||||
Table 3: The antifungal activity of fungicidal synthesized compounds.
Antimalarial evaluation
All the newly synthesized compounds (6a-6e) were screened for their in vitro antimalarial activity against the human malarial causative strain Plasmodium falciparum and were compared with standard drugs gentamycin, ampicillin, chloramphenicol, ciprofloxacin, and norfloxacin. The antimalarial activity data suggested that compound 6a exhibited maximum inhibition against Plasmodium falciparum with IC50 values 0.05 μg/mL respectively among all in comparison to quinine. Compound 6a was significantly active while the rest of the compounds were moderate in antimalarial activity [15].
Procedure: The in vitro antimalarial assay was carried out in 96 microtiter plates according to the microassay protocol of Rieckmann and coworkers with minor modifications. The culture of Plasmodium falciparum strain was maintained in medium RPMI 1640 supplemented with 25 Mm HEPES, 1% D-glucose, 0.23% sodium bicarbonate, and 10% heat-inactivated human serum. Then synchronous parasites of P. falciparum were synchronized after 5% D-sorbitol treatment to obtain only the ring-stage parasitized cells. For carrying out the assay, an initial ring stage parasitemia of 0.8% to 1.5% hematocrit in a total volume of 200 μ one of medium RPMI-1640 was determined by Jaswant Singh Bhattacharya (JSB) staining to assess the percent parasitemia (rings) and uniformly maintained with 50% RBCs (O+). A stock solution of 5 μmg/ml of each of the test samples was prepared in DMSO and subsequent dilutions were prepared with a culture medium. The diluted samples in 20 μl volume were added to the test samples to obtain a final concentration ranging between 0.4 g/ml in a duplicate well containing parasitized cell preparation. The culture plates were incubated at 37°C in a candle jar. After 36-40 hours of incubation, a thin blood smear from each sample was prepared and stained with JSB stain. Then slides were microscopically observed (Table 4) [15-18].
|
Minimum inhibitory concentration |
||
|
Sr.no |
Compound ID |
Mean IC50 values |
|
1 |
6a |
0.72 µg/ml |
|
2 |
6b |
1.16 µg/ml |
|
3 |
6c |
0.86 µg/ml |
|
4 |
6d |
0.81 µg/ml |
|
5 |
6e |
0.95 µg/ml |
|
6 |
Chloroquine |
0.020 µg/ml |
|
7 |
Quinine |
0.268 µg/ml |
Table 4: The antimalarial activity of Plasmodium falciparum synthesized compounds.
The anthelmintic evaluation was done against Pheretima posthuma. Anthelmintic activity was determined by calculating the mean paralysis time and mean death time. The anthelmintic activity of the entire compound was compared with a standard drug (albendazole). All synthesized compounds exhibited good anthelmintic activity.
• The standard drug (Albendazole) demonstrated significant activity (14.4 ± 13.96) at mean paralysis time among thesynthesized compound 6b (17.4 ± 15.6) and compound 6f showed moderate activity (19.8 ± 19.2).
• The standard drug (Albendazole) demonstrated significant activity (27.4 ± 26.92) at mean death time among the synthesizedcompound 6b (27.6 ± 26.96) and compound 6f showed moderate activity (15.4 ± 14.93).
The antibacterial evaluation was determined by minimal inhibition concentration against bacteria Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes. The antibacterial activity of the test compounds was also compared against the standard drug. Most of the synthesized compounds exhibited significant antibacterial activity (Figure 5).
•The compound 6b, 6d, 6h, 6i, showed potent activity against Escherichia coli in comparison to standard drug ampicillin, andcompound 6f showed potent activity and 6g and 6j showed moderate activity in comparison to standard drugchloramphenicol.
•The compounds 6d, 6f, 6g, 6i, and 6j showed potent activity against Pseudomonas aeruginosa in comparison to the standarddrug ampicillin.
•The compound 6c, 6h showed potent activity against Staphylococcus aureus in comparison to the standard drug ampicillin.
•The compounds 6c, 6d, 6f, 6g, 6h, and 6j showed potent activity against Streptococcus pyogenes in comparison to thestandard drug ampicillin.
Figure 5: Column graph of the antibacterial activity of 6a-6j.
Antifungal evaluation
The antifungal evaluation was done by Agar well diffusion method against bacteria and Candida albicans. Antifungal activity was determined by minimum fungal concentration. The antifungal activity of the test compounds was also compared against the standard drug. Most of the synthesized compounds exhibited significant antifungal activity (Figure 6).
•The compound 6b, 6h showed potent activity against Candida albicans in comparison to the standard drug griseofulvin.
•The compounds 6d, 6e, 6f, 6g, 6h, and 6j showed potent activity against Aspergillus niger in comparison to the standarddrug griseofulvin.
•The compounds 6d, 6e, 6g, and 6h, 6j showed potent activity against Aspergillus clavatus in comparison to the standard druggriseofulvin.
•The compounds showed moderate activity against bacteria in comparison to the standard drug nystatin.
Figure 6: Column graph of the antifungal activity of synthesized compounds 6a-6j.
Antimalarial evaluation malaria, which is usually caused by protozoan parasites of the genus Plasmodium including Plasmodium falciparum, species of the human malaria parasite, is one of the most widespread and deadliest diseases throughout the world (Figure 7).
•The compound 6a was most potent against Plasmodium falciparum with IC50 of 0.72 μg/ml respectively among all incomparison to the quinine.
•Compound 6a was significantly active while the rest of the compounds showed moderate activity to the chloroquine.
Figure 7: The antimalarial activity of synthesized compounds.
Hydrazone compounds are a key class of molecules for the development of new medicines, to identify an efficient compound in fighting multidrug-resistant bacterial infections. These compounds possess diverse biological and pharmacological properties such as antimicrobial, anti-inflammatory, analgesic, antifungal, anti-tubercular, antiviral, anticancer, antiplatelet, antimalarial, anticonvulsant, cardio-protective, anthelmintic, antiprotozoal, anti-trypanosomal, anti-schistosomiasis, etc. These compounds contain a C=N bond, which is conjugated with a lone pair of electrons of the functional nitrogen atom.
Pyrimidine as an important constituent of nucleic acids, pyrimidine has been occupying a distinct and unique place in human life and consequently in organic and medicinal chemistry research. Although, the moiety is mainly known for its biological significance, its importance in the field of material chemistry can never be underestimated as lots of pyrimidine derivatives are reported recently as a constituent of liquid crystal, fluorescence material, photo-responsive surfaces, etc.
Chemistry
The unsymmetrical substituted key precursor, 1-(-4-methyl-6-phenylpyrimidin-2-yl) hydrazine was initially prepared by a series of reactions as shown in Figure 8. Commercially available benzoyl acetone was refluxed in urea first in methanol for 15 minutes and further for 24 hours in the presence of 1 equivalent concentrated HCL to produce 4-methyl-6-phenylpyrimidin-2-ol in a 56% yield. Subsequently, refluxing with phosphorus oxychloride to give 2-chloro-4-methyl-6-phenylpyrimidine which on refluxing with hydrazine hydrate for 3-5 hours underwent nucleophilic reaction and resulted in required 1-(-4-methyl-6-phenylpyrimidin-2-yl)hydrazine. The synthesis of hydrazones bearing unsymmetrically substituted pyrimidine group (6a-6j) in different yields was achieved by a condensation reaction of 1-(-methyl-6-phenylpyrimidin-2-yl)hydrazine (1.0 equivalent) with various benzaldehyde derivatives (1.0 equivalent) in refluxing ethanol using or having a presence of the catalytic amount of glacial acetic acid.
Spectral analysis
The spectral explication of pyrimidine hydrazones (6a-6j) was ascertained based on their detailed spectroscopic data (IR, 1H NMR) analysis. The characteristic NH peak for hydrazones (6a-6j) appeared in the region of 3500-3100 cm-1. Evanescence of singlet of áµ?4.10 ppm (NH2) and slight downfield in signals at (NH) and appearance of new characteristics signals in region áµ?ppm and áµ?ppm as singlet strongly indicate the presence of NH group respectively in hydrazones (6a-6j). For the pyrimidine core, the hydrogen at C5 appeared as a sharp singlet at áµ?7.48-7.21 ppm and three protons correspond to the CH3 group at C4 resonates in between áµ?3.10-2.85 ppm as singlet peak. All remaining protons accredited with phenyl rings appeared in their expected aromatic region.
Heterocyclic systems are found in several drugs, as well as biotechnologically important molecules. The presence of homoatoms or groupings gives them preferential characteristics in their biological responses. Benzaldehyde is a very important part of heterocyclic systems, which plays a fundamental role in organic and medicinal chemistry.
Due to their potent and significant biological activities, these agents have great pharmaceutical importance, hence their synthesis is of considerable interest. In compounds used for research intended to test new products that have exciting biological activities, the nucleus of benzaldehyde is present. Benzaldehyde is a family of chemicals whose chemical skeleton is constituted of a benzene ring fused with a ring. It is used in a variety of products and operations, being manufactured all over the world. Depending on the substituents on the benzaldehyde moiety, they can have a wide range of biological properties and can be used as cosmetic products.
Hydrazones constitute chemical entities, which are a vivid area of pharmaceutical, and drug candidates and spacious impedance as outstanding precursors for the discovery of numerous heterocyclic compounds. In addition, hydrazones pharmacophores are associated with profound biological activities, such as anticancer, anti-inflammatory, antifungal, insecticidal, antioxidant, and antitubercular. Amongst the heterocyclic system, benzaldehyde is a biologically important scaffold known to be associated with several biological activities. Some of the prominent biological responses attributed to this skeleton are anticancer, antituberculosis, antimalarial, and anthelmintic activities. Many researchers have been drawn to the diversity in the biological response profiles of benzaldehyde due to its many potentials against a variety of activities. Hence the biological significance of benzaldehyde along with the pharmacological importance of hydrazine motivated us to synthesize some new benzaldehyde derivatives using pyrimidine as a starting material. The target compounds were synthesized taking pyrimidine as a starting material. In the first, 1-(-4-methyl-6-phenylpyrimidin-2-yl)hydrazine was initially prepared by a series of reactions. Commercially available benzoyl acetone was refluxed in urea first in methanol for 15 minutes and further for 24 hours in the presence of 1 equivalent conc. HCL to produce 4-methyl-6-phenylpyrimidin-2-ol in 56% yield. Subsequently, refluxing with phosphorus oxychloride to give 2-chloro-4-methyl-6-phenylpyrimidine which on refluxing with hydrazine hydrate for 3-5 hours undergoes nucleophilic reaction and resulted in required 1-(-4-methyl-6-phenylpyrimidin-2-yl)hydrazine. The synthesis of hydrazones bearing unsymmetrically substituted pyrimidine group in different yields was achieved by condensation reaction of 1-(-methyl-6-phenylpyrimidin-2-yl)hydrazine (1.0 equivalent) with various benzaldehyde derivatives (1.0 equivalent) in refluxing ethanol using or having a presence of a catalytic amount of glacial acetic acid. The structures of all the newly synthesized compounds were confirmed by suitable spectroscopic methods such as IR, 1H NMR spectral data. Keeping in view the activity profile of benzaldehyde and hydrazones themselves, it was thought to carry out the pharmacological screening of all the newly prepared heterocyclic compounds. Therefore all the synthesized compounds were subjected to anthelmintic, anti-bacterial, anti-fungal, and antimalarial evaluation.
Experimental studies
General procedure for the synthesis of different benzaldehyde derivative compounds: The unsymmetrical substituted key precursor, 1-(-4-methyl-6-phenylpyrimidin-2-yl) hydrazine was initially prepared by a series of reactions as shown in Figure 8. Commercially available benzoyl acetone 1 was refluxed in urea first in methanol for 15 minutes and further for 24 hours in the presence of 1 equivalent concentrated HCL to produce 4-methyl-6-phenylpyrimidin-2-ol 2 in a 56% yield. Subsequently, refluxing of 2 with phosphorus oxychloride to give 2-chloro-4-methyl-6-phenylpyrimidine 3 which on refluxing with hydrazine hydrate for 3-5 hours underwent nucleophilic reaction and resulted in required 1-(-4-methyl-6-phenylpyrimidin-2-yl)hydrazine.
Figure 8: Synthetic pathways for 1-(4-methyl-6-phenylprimidin-2-yl)hydrazine.
The synthesis of hydrazones bearing unsymmetrically substituted pyrimidine group (6a-6j) in different yields was achieved by a condensation reaction of 1-(-methyl-6-phenylpyrimidin-2-yl)hydrazine 4 (1.0 equivalent) with various benzaldehyde derivatives 5 (1.0 equivalent) in refluxing ethanol using or having a presence of the catalytic amount of glacial acetic acid (Figure 9) [5].
Figure 9: Synthetic pathways for 2-arylidine-1-(2-methyl-quinoxalin-3-yl)hydrazine.
Steps for the synthesizing compounds
Step 1: Procedure for synthesis of 4-methyl-6-phenyl pyrimidine-2-ol: Benzoyl acetone was added to the hot solution of urea (0.7 g) in methanol (35 ml) and refluxed. After refluxing (60°C) the resulting mixture for 15-20 minutes, concentrated hydrochloric acid was added slowly. The combined mixture was further refluxed for 8 hours and subsequently allowed to cool overnight at room temperature when needle-shaped crystals of 2-hydroxy-4-methyl-6-phenyl pyrimidine from hydrochloric salt were separated. Afterward, hydrochloride salts thus obtained were dissolved in a minimum amount of water and neutralized with a concentrated sodium bicarbonate solution. Immediate formation of precipitate took place. Further kept overnight and filtered off to give pure 4-methyl-6-phenylpyimidin-2-ol (1 g), off-white solid, m.p. (228°C-230°C).
Step 2: Procedure for the synthesis of 2-chloro-4-methyl-6-phenyl pyrimidine: The reaction was carried out by refluxing 2-hydroxy-4-methyl-6-phenylpyrimidine with phosphorus oxychloride (10 ml) for 4-5 hours. After completion of the reaction (monitored by TLC) excess phosphorus, and oxychloride were removed under reduced pressure. Afterward, the brown gummy mass was obtained, then poured into ice-cold water, and neutralized by sodium bicarbonate solution. The light brown precipitate was filtered off, then dried and identified as 2-chloro-4-methyl-6-phenylpyrimdine, after ethanol recrystallization Rf-0.67 m.p: (58°C-60°C).
Step 3: Procedure for the synthesis of 2-hydrazinyl-4-methyl-6-phenylpyrimidine: 2-Chloro-4-methyl-6-phenylpyrimidine (1.75 g) was refluxed with an excess of hydrate in ethanol (20 ml) as a solvent for 2 hours to afford 2-hydrazinyl-4-methyl-6-phenylpyrimidine Rf-0.10, m.p: (132°C-134°C).
Step 4: Procedure for synthesis of 2-arylidene-1-(4-methyl-6-phenylpyrimidine-2-yl) hydrazine: To the ethanolic solution of 2-hydrazinyl-4-methyl-6-phenylpyrimidine in a round bottom flask, benzaldehyde was added, and the solution was refluxed for 1hour with (2-3 drops) of glacial acetic acid (Table 5 and Figure 6). The solvent was evaporated to half of its volume and cooled atroom temperature. The solid obtained was filtered and further purified by recrystallization from ethanol to get the targetcompound i.e., 2-arylidene-1-(4-methyl-6-phenylpyrimidine-2-yl)hydrazine (Tables 6 and 7).
|
Compounds |
Compounds name |
Molecular formula |
Quantity used (g) |
|---|---|---|---|
|
5a |
2,4-Dimethoxy benzaldehyde |
C9H10O3 |
1.0 |
|
5b |
4-Methoxy benzaldehyde |
C8H8O2 |
1.0 |
|
5c |
2-Chloro benzaldehyde |
C7H5ClO |
0.95 |
|
5d |
2-Fluoro benzaldehyde |
C7H5FO |
1.0 |
|
5e |
4-Bromo benzaldehyde |
C7H5BrO |
1.0 |
|
5f |
2,4-Dichloro benzaldehyde |
C7H4Cl2O |
0.85 |
|
5g |
2-Furfuraldehyde |
C5H4O2 |
1.0 |
|
5h |
2-Thiophene carboxaldehyde |
C5H4OS |
1.0 |
|
5i |
Cinnamaldehyde |
C9H8O |
0.95 |
|
5j |
3-Ethoxy-4-hydroxy benzaldehyde |
C9H10O3 |
1.0 |
Table 5: Benzaldehyde is used for synthesizing compounds.
Figure 10: Structure of synthesized compound 2-arylidene-1-(4-methyl-6-phenylpyrimidine-2-yl)hydrazine.
| Compounds | Molecular formula | Molecular weight* (g) | Melting point °C | Rf value | % Yield | Color | Odor |
|---|---|---|---|---|---|---|---|
| 6a | C20H20N4O2 | 348.4 | 132-134°C | 0.36 | 70% | Off white | Vanilla |
| 6b | C21H20N4O4 | 392.4 | 130-134°C | 0.6 | 80% | Pale yellow | Sweet woody |
| 6c | C18H15ClN4 | 338.7 | 174-179°C | 0.7 | 68% | Off white | Vanilla beans |
| 6d | C18H15FN4O | 322.4 | 165-167°C | 0.55 | 85% | Yellow | Anise |
| 6e | C18H15BrN4O | 383.2 | 165-168°C | 0.81 | 75% | Clear colorless to yellow | Tobacco |
| 6f | C18H14Cl2N4O | 373.2 | 117-120°C | 0.45 | 60% | White | Pungent |
| 6g | C16H14N4O2 | 294.31 | 130-134°C | 0.51 | 65% | White crystalline solid | Pungent |
| 6h | C16H14N4OS | 310.3 | 133-135°C | 0.23 | 90% | Off white | Sweet woody almond |
| 6i | C2OH21N5O | 347.4 | 131-133°C | 0.81 | 80% | Yellow | Fruity type |
| 6j | C21H22N4O3 | 378.42 | 170-173°C | 0.72 | 85% | Clear colorless to yellow | Almond odor |
| *Calculated value | |||||||
Table 6: Structural and physicochemical data of synthesized compounds.
Spectral characterization of synthesized compounds
| S. no | Synthesized compounds | Spectral ranges |
|---|---|---|
| 1. | (E)-2-(2,4-dimethoxybenzylidene)-1-(4-methyl-6-phenylpyrimidin-2-yl)hydrazine (6a)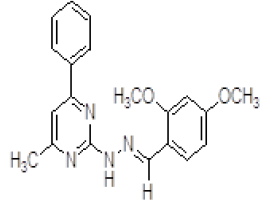 |
1H NMR (DMSO áµ?ppm): 9H-7.481-4.801 (CH aromatic), 2H-4.010 (s, NH hydrazine), 6H-3.339 (s, 2CH3), 3H-2.351 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3337 (N-H structure), 3050 (C-H structure, 2819 (C-H), 1178 (C-N structure). |
| 2. | (E)-2,4-methoxy-1-(4-methyl-6-phenylpyrimidin-2-yl)hydrazine (6b)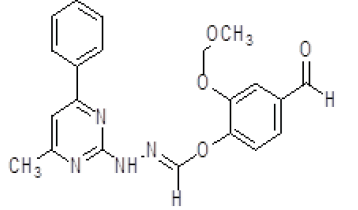 |
1H NMR (DMSO áµ?ppm): 9H-7.481-6.803 (CH aromatic), 3H-7.340 (t,CH), 2H-7.034-6.011 (s, NH), 3H-3.341 (s, CH3), 1H-3.241 (s, CH), 3H-2.511 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3501 (N-H structure), 3070 (C-H structure), 2870 (C-NCH3), 1266 (C-N structure). |
| 3. | 2-Chloro-N'-(4-methyl-6-phenylpyrimidin-2-yl)benzohydrazine (6c)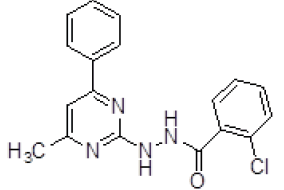 |
1H NMR (DMSO áµ?ppm): 2H-8.012-4.010 (s, NH hydrazine), 10H-7.481-6.591 (CH aromatic), 3H-2.511 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3469 (N-H structure), 3010 (C-H structure), 2840 (C-CH3), 1213 (C-N structure). |
| 4. | 2-Fluoro-N'-(4-methyl-6-phenylpyrimidin-2-yl)benzohydrazine (6d)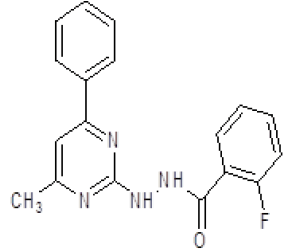 |
1H NMR (DMSO áµ?ppm): 2H-8.212-4.021 (s, NH hydrazine), 10H-7.471-6.594 (CH aromatic), 3H-2.511 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 2782 (N-H structure), 2661 (C-CH3), 1623 (C=N), 1155 (C-N structure). |
| 5. | 2-Bromo-N'-(4-methyl-6-phenylpyrimidin-2-yl)benzohydrazine (6e)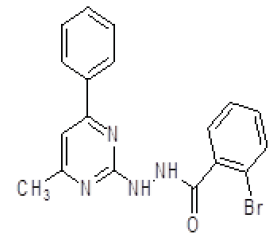 |
1H NMR (DMSO áµ?ppm): 2H-8.242-4.121 (s, NH hydrazine), 10H-7.451-6.424 (CH aromatic), 3H-2.531 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3368 (C-H structure), 2836 (C-CH3), 1623 (C=N), 1315 (C-N structure). |
| 6. | 2,4-Dichloro-N'-(4-methyl-6-phenylpyrimidin-2-yl)benzohydrazine (6f)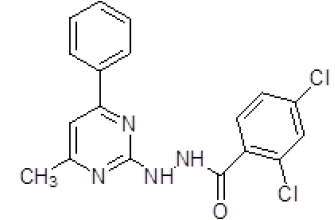 |
1H NMR (DMSO áµ?ppm): 9H-7.462-6.801 (CH aromatic), 2H-4.210 (s, NH hydrazine), 3H-2.153 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3419 (N-H structure), 2999 (C-H structure), 2915 (C-CH3 structure), 1660 (C=N), 1312 (C-N structure). |
| 7. | N'-(4-methyl-6-phenylpyrimidin-2-yl)furan-2-carbohydrazide (6g)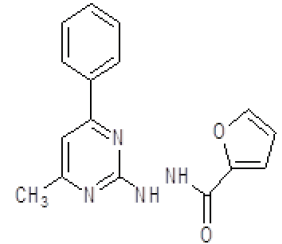 |
1H NMR (DMSO áµ?ppm): 6H-7.481-4.801 (CH aromatic), 2H-3.332 (s, NH hydrazine), 3H 7.261-6.661 (s, CH), 3H-2.231 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 2236 (C-H structure), 2217 (C-CH3 structure), 1623 (C=N), 1080 (C-N structure). |
| 8. | N'-(4-methyl-6-phenylpyrimidin-2-yl)thiophene-2-carbohydrazide (6h)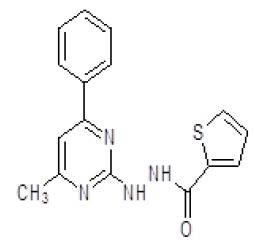 |
1H NMR (DMSO áµ?ppm): 6H-7.471-4.601 (CH aromatic), 2H-4.332 (s, NH hydrazine), 3H 7.231-6.261 (s, CH), 3H-2.131 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 2782 (N-H structure), 2661 (C-CH3 structure), 1623 (C=N), 1155 (C-N structure). |
| 9. | 4-(Dimethylamino)-N-(4-methyl-6-phenylpyrimidin-2-yl)benzohydrazine (6i)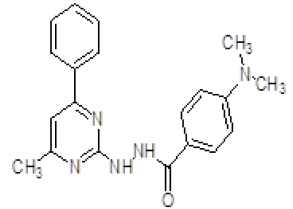 |
1H NMR (DMSO áµ?ppm): 10H-7.331-6.803 (CH aromatic), 2H-7.034-6.011 (s, NH), 6H-2.853-2.341 (s, CH3), 3H-2.511 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3048 (C-H structure), 3102 (N-H structure), 2831 (C-CH3 structure), 1620 (C=N), 1097 (C-N structure). |
| 10. | 3-Ethoxy-4-methoxy(4-methyl-6-phenylpyrimidin-2-yl)benzohydrazine (6j)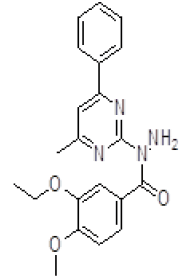 |
1H NMR (DMSO áµ?ppm): 9H-7.351-6.203 (CH aromatic), 2H-6.034-4.011 (s, NH), 3H-7.351-6.840 (s, CH), 3H-7.210-7.011 (s, CH), 3H-2.511 (s, CH3 pyrimidine). IR (KBr) v (cm-1): 3100 (N-H structure), 2778 (C-H structure), 2562 (C-CH3 structure), 1660 (C=N), 1011 (C-N structure). |
Table 7: Spectral analysis of synthesized compounds.
In this view, we have attempted to review the literature on the hydrazine, pyrimidine, and benzaldehyde compounds for their medicinal significance with the help of chemical abstracts, articles, journals, and internet sites. The literature review and survey were carried out in 2000 to update the literature on hydrazine, pyrimidine, and benzaldehyde compounds. In light of the above, the synthesis of new compounds was established in the literature survey. A total of 10 compounds were synthesized. The yields of different synthesized compounds were found to be under different ranges. The synthesized compounds were characterized by spectral analysis (IR and 1H NMR). The array of novel derivatives was evaluated by antimicrobial activity against bacterial strains. Preliminary results revealed that most of the synthesized compounds demonstrated good activities in gram-negative and gram-positive bacterial strains. In the present investigation, we describe the synthesis and anthelmintic, antibacterial antifungal, and antimalarial evaluation of novel derivatives of selected drugs. The major findings indicated that most of the synthesized compounds were equally active against gram-positive rather than gram-negative bacteria. Compounds 6a and 6e were observed to be the most potent compound.
In synthesized compounds, 6g, and 6h demonstrated potent antifungal activity and 6a was observed more potent in the antimalarial activity. Among all synthesized compounds, compounds 6b and 6g demonstrated potent anthelmintic activity with mean paralysis time ranging from 17.4 ± 15.6 min and 9.8 ± 9.2 min, death time ranging from 27.6 ± 26.96 min and 9.8 ± 9.2 min as compared to standard drug.
The Supporting information contains detailed synthetic as well as experimental procedures, compound data and characterization, spectra (IR, 1HNMR,) and references.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Gupta S, et al. 2025. Synthesis and Evaluation for Anti-Infective Activities of 2-Arylidene-1-(4-Methyl-6- Phenylpyrimidin-2-YL) Hydrazides. Der Pharma Lett.17:01-13.
Copyright: © 2025 Gupta S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.